The tablet compression machine stands as a cornerstone of modern pharmaceutical manufacturing, enabling the precise and efficient production of tablets that meet stringent quality standards. Its operation involves a sophisticated interplay of mechanical, electrical, and hydraulic systems, designed to convert fine powders into uniform, compact tablets. This article provides an in-depth examination of the core working principles behind this essential equipment, shedding light on the processes and mechanisms that ensure optimal functionality. Whether you are a pharmaceutical engineer, a quality specialist, or an enthusiast looking to expand your technical knowledge, this guide is designed to demystify the complexities of tablet compression technology. Read on to gain valuable insights into how these machines operate, the critical components involved, and the science that drives their performance.
What Is a Compression Machine and How Does It Work?

A compression machine, commonly referred to as a tablet press, is a device used to compress powder or granules into solid tablets of uniform size and weight. It operates by applying high pressure between two punches and a die to shape the material into a compact form. The process begins with the material being fed into the die cavity, where it is evenly distributed. The punches then exert force from both above and below the die to compress the material into a tablet. This ensures consistency in both density and dosage, making the machine a critical tool in pharmaceutical manufacturing and other industries requiring precise tablet production.
Exploring the Principle Behind Tablet Compression
The principle of tablet compression revolves around the application of mechanical force to convert granulated or powdered material into a solid, uniformly compacted form. This is achieved through a carefully orchestrated blend of processes, including filling, compression, and ejection. The first stage ensures the die cavity is uniformly filled to avoid inconsistencies in tablet weight. During compression, upper and lower punches exert significant pressure to consolidate the material, creating a dense and cohesive structure. Key variables influencing this process include punch speed, compression force, and formulation properties. Optimal calibration of these variables ensures the uniformity, hardness, and stability of the tablets, meeting stringent regulatory standards essential in pharmaceutical applications. Finally, the ejection stage involves the release of the tablet from the die without structural damage, highlighting the precision of the entire operation.
Key Components of a Tablet Compression Machine
- Hopper
The hopper is responsible for holding and feeding the granulated powder mixture into the die cavity. This component ensures consistent material flow, minimizing variability in tablet weight. Optimal hopper design incorporates a flow rate control mechanism, reducing the risk of segregation.
- Feed System
The function of the feed mechanism is to transfer the material from the hopper into the die cavity efficiently. Common designs include gravity-fed and force-fed systems, enabling precise control over material flow based on formulation requirements.
- Punches (Upper and Lower)
The punches compress the powder within the die cavity to form a tablet. Critical parameters include punch speed, which typically ranges from 30–60 RPM for single-station presses, and compression force, which varies from 5–30 kN for standard applications and up to 100 kN for specialized formulations like effervescent tablets.
- Die Cavity
The die cavity determines the shape and size of the final tablet. Die design must conform to tablet specifications, typically ranging from 5–25 mm in diameter, depending on dosage requirements and intended use.
- Cam Tracks
These tracks guide the motion of punches during the compression cycle. Proper calibration of cam tracks ensures precision in de-aeration, compression, and ejection stages. Misaligned tracks can lead to tablet defects such as capping or lamination.
- Compression Rollers
Compression rollers apply force to the punches, yielding the desired tablet density and hardness. Key parameters include pre-compression force (2–10 kN) and main compression force, which should be validated to ensure compliance with target mechanical properties.
- Ejection Mechanism
The ejection mechanism facilitates the smooth removal of tablets from the die cavity. It relies on a combination of lower punch motion and anti-stick coatings on tooling surfaces to avoid structural damage.
- Control System
Modern tablet compression machines are equipped with advanced control systems for monitoring and adjusting variables such as feed rate, punch force, and machine speed in real time. These systems ensure seamless operation while meeting production and quality standards outlined in GMP (Good Manufacturing Practices).
Accurate configuration and maintenance of these components are essential to achieving highly uniform and defect-free tablets in pharmaceutical manufacturing.
The Role of Hydraulic Pressure in Tablet Pressing
Hydraulic pressure plays a critical role in the tablet pressing process, influencing several key factors that directly impact product quality and consistency. These roles include:
- Compression Force Control: Hydraulic systems regulate the pressure applied during the compression phase, ensuring tablets achieve the desired hardness and density without compromising structural integrity.
- Uniform Weight Distribution: By maintaining consistent pressure across the die cavity, hydraulic systems help achieve uniform tablet weight and reduce variation in batches.
- Minimization of Tablet Defects: Adequate hydraulic pressure prevents common defects such as capping, lamination, or cracking by ensuring optimal material compaction.
- Adjustment for Material Characteristics: Hydraulic systems can be fine-tuned to accommodate varying material flow properties, ensuring consistent performance across formulations with differing granule properties.
- System Stability and Energy Efficiency: By using precisely controlled hydraulic systems, the tablet press operates more efficiently with reduced wear on mechanical components, enhancing both machine lifespan and energy use optimization.
Accurate calibration and monitoring of hydraulic pressure are imperative to uphold manufacturing standards and ensure the production of high-quality pharmaceutical tablets.
How to Perform a Compression Test on Tablets
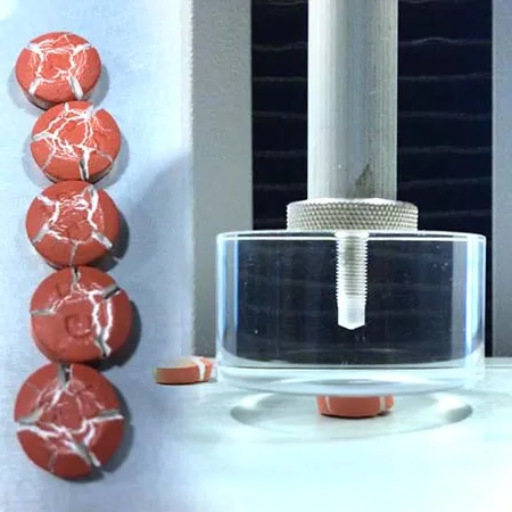
Step-by-Step Guide to Performing a Compression Test on Tablets
- Preparation of Equipment
Begin by ensuring the tablet compression testing machine is clean and properly calibrated. Verify that all necessary components, such as the load cell and platens, are functioning correctly to ensure accurate readings.
- Sample Selection
Collect a representative sample of tablets from the production batch. The sample size should comply with regulatory guidelines to provide statistically significant results.
- Tablet Placement
Position a single tablet on the testing platform of the compression tester. Ensure it is centrally aligned to prevent variations in the force application.
- Parameter Setup
Input test parameters into the machine, including the desired compression force range and speed in accordance with the tablet specifications and industry standards.
- Conducting the Test
Initiate the test by applying compressive force to the tablet. The machine will measure and record the peak force required to fracture or deform the tablet.
- Recording Results
Document the measured values, such as the breaking force (in Newtons), and compare the data against product design specifications and regulatory requirements.
- Analysis and Reporting
Analyze the test results to evaluate the mechanical strength and overall quality of the tablets. Identify any anomalies or deviations and report the findings for further quality assurance investigation.
Accurate execution of each of these steps is crucial to ensuring the reliability of the compression test and maintaining consistency in pharmaceutical tablet production.
Setting Up a Compression Testing Machine
To set up a compression testing machine accurately, I first ensure the equipment is placed on a stable and level surface to minimize vibration or external interference during operation. Next, I verify that all components, such as the loading platform, grips, and alignment fixtures, are properly assembled and securely tightened based on the manufacturer’s guidelines. I then power on the machine and configure the control settings, including selecting the correct load cell and calibration mode for the desired testing parameters. After loading a test sample, I check the alignment to confirm that the axis of the load application matches the machine’s load cell alignment, ensuring precision in force measurement. Finally, I perform a short trial run to validate that the system operates smoothly and outputs data accurately before proceeding with actual sample testing.
Steps to Determine a Material’s Behavior Under Pressure
- Prepare the Testing Equipment
Ensure that the testing machine is fully operational, calibrated, and configured with the appropriate load cell and settings based on the material being tested.
- Select and Prepare the Test Sample
Cut or shape the sample material to meet the required dimensions and specifications for accurate testing, ensuring surface quality and uniformity where applicable.
- Align the Sample and Equipment
Mount the sample within the testing machine, verifying that it is aligned precisely to the axis of force application to prevent bending or asymmetric loading.
- Calibrate the System
Perform a calibration of both the machine and the sensors to guarantee precise force, displacement, or stress measurements during the test.
- Set Testing Parameters
Program the testing machine with critical parameters, such as load rate, temperature range (if applicable), and desired testing mode (e.g., tension, compression, or shear).
- Conduct a Trial Run
Execute a short preliminary run to confirm that all components operate as intended, that data recording systems function correctly, and that no misalignment or equipment malfunctions occur.
- Perform the Test
Apply stress or force to the sample according to the specified parameters, observing and recording material responses like deformation, fracture, or failure.
- Analyze the Data
Compare the recorded data against theoretical models or material standards to determine mechanical properties such as yield strength, tensile strength, elasticity, or ductility.
- Document Results
Compile a detailed report of the test methodology, raw data, and findings, ensuring that the results can be replicated or referenced for future studies.
- Inspect Equipment Post-Test
After testing, inspect the machinery for any signs of wear, misalignment, or potential damage, and re-calibrate if necessary to maintain accuracy for subsequent tests.
What is the Difference Between Pneumatic and Hydraulic Compression Machines?

Pneumatic compression machines use compressed air as the driving force, while hydraulic compression machines employ pressurized hydraulic fluid, leading to differences in power, precision, maintenance, and applications.
|
Parameter |
Pneumatic |
Hydraulic |
|---|---|---|
|
Driving Force |
Compressed Air |
Hydraulic Fluid |
|
Power Output |
Lower |
Higher |
|
Precision |
Moderate |
High |
|
Speed |
Faster |
Slower |
|
Durability |
Less Durable |
More Durable |
|
Maintenance |
Easier |
Complex |
|
Cost |
Lower |
Higher |
|
Applications |
Light Duty |
Heavy Duty |
Understanding Pneumatic Compression Devices
Pneumatic compression devices are medical tools designed to improve circulation and prevent conditions such as deep vein thrombosis (DVT) and chronic venous insufficiency. By utilizing air-driven cuffs that sequentially inflate and deflate around limbs, these devices promote venous blood return to the heart, reduce swelling, and enhance lymphatic drainage. They operate by creating intermittent pneumatic pressure, mimicking the natural muscle contractions that assist blood flow.
There are three primary components in these systems: an air pump or compressor, inflatable sleeves or cuffs, and connecting hoses. The air pump generates and regulates pressure, while the cuffs are strategically placed to target specific areas, such as legs or arms. Modern devices often incorporate programmable settings, allowing for tailored pressure levels and inflation cycles based on patient requirements.
Pneumatic compression devices are widely used in hospitals, outpatient clinics, and home care settings. They are particularly beneficial in post-surgical recovery and for individuals at risk of blood clots or edema. However, these devices are contraindicated in patients with certain conditions such as severe arterial insufficiency or active infections. Proper medical oversight is essential for safe and effective application.
Comparing Hydraulic and Pneumatic Systems
Hydraulic and pneumatic systems differ primarily in their mediums, energy efficiency, precision, cost, and applications.
|
Parameter |
Hydraulic |
Pneumatic |
|---|---|---|
|
Medium |
Liquid |
Gas |
|
Pressure |
High |
Low |
|
Energy Eff. |
Efficient |
Less eff. |
|
Power |
High output |
Lower output |
|
Precision |
High |
Moderate |
|
Speed |
Slow |
Fast |
|
Cost |
Expensive |
Affordable |
|
Maintenance |
Complex |
Easy |
|
Applications |
Heavy-duty |
Lightweight |
|
Noise |
Quiet |
Noisy |
Applications in Compression Therapy
- Treatment of venous insufficiency and varicose veins
- Management of lymphedema
- Reduction of swelling and edema in post-surgical patients
- Promoting blood flow and preventing deep vein thrombosis (DVT)
- Aiding in muscle recovery for athletes
- Alleviation of symptoms associated with chronic venous disorders
- Support in wound healing by increasing oxygenated blood circulation
The Pharmaceutical Applications of Tablet Compression Machines

Tablet compression machines are fundamental in the pharmaceutical industry for the production of uniform, high-quality tablets. They are employed in the mass manufacturing of medicinal tablets by compressing powdered or granular materials into solid, defined shapes. These machines ensure precise dosage accuracy, which is critical for therapeutic efficacy and patient safety. Additionally, they are capable of producing tablets with varying pharmacological requirements, including immediate-release, extended-release, or controlled-release formulations. Advanced tablet compression systems also facilitate multi-layered tablet production, enabling the combination of active ingredients or controlled release profiles in a single dosage form. Their efficiency and adaptability make them indispensable for modern pharmaceutical manufacturing processes.
Importance of Tablet Press in Medicine Production
Tablet presses play a critical role in medicine production by ensuring accurate dosage, consistency, and efficiency in large-scale manufacturing. The precise control over compression force and fill volume enables the production of tablets with uniform weight, hardness, and disintegration properties, meeting stringent regulatory standards. Advanced tablet press systems are equipped with automated controls to monitor pressure, turret speed, and filling depth, ensuring high precision and minimal variability.
Key Technical Parameters of Tablet Presses:
- Compression Force: Typically ranges from 5 to 100 kN, depending on the tablet type (e.g., immediate-release or extended-release).
- Turret Speed: Adjustable from 10 to 100 RPM to optimize production efficiency without compromising quality.
- Filling Depth: Often set between 2 to 20 mm to control the tablet’s weight and size.
- Tablet Hardness: Achievable hardness values between 2 to 20 kp, ensuring mechanical stability and appropriate disintegration times.
- Output Capacity: Modern tablet presses can produce 5,000 to 1,000,000 tablets per hour, depending on the model and application.
These technical parameters not only ensure the production of high-quality tablets but also facilitate compliance with pharmaceutical industry standards such as GMP (Good Manufacturing Practice). By leveraging such precision machinery, manufacturers achieve superior efficiency and maintain the trust of healthcare providers and patients.
Achieving Consistency in Pharmaceutical Manufacturing
Ensuring Uniformity in Pharmaceutical Manufacturing Processes
Consistency in pharmaceutical manufacturing is achieved through stringent adherence to regulatory guidelines, precise control of processes, and the integration of advanced technologies. Key approaches include implementing robust Quality Management Systems (QMS), such as Good Manufacturing Practice (GMP), which enforce standard operating procedures across all production stages. Additionally, automated systems and real-time monitoring technologies play a pivotal role by minimizing human error and enabling precise control over critical parameters like temperature, pressure, and ingredient ratios.
Process validation is another critical component, involving rigorous testing to ensure reproducibility and reliability across production batches. Analytical tools, including spectroscopy and chromatography, are extensively employed to verify the chemical and physical properties of pharmaceutical products, ensuring they meet strict quality specifications. Furthermore, data integrity is maintained through digital record-keeping systems, which provide traceability and accountability throughout production and distribution. By adopting these measures, pharmaceutical manufacturers not only achieve consistency but also uphold public health and compliance with regulatory bodies worldwide.
Common Issues and Troubleshooting in Tablet Compression Machines

Common Issues in Tablet Compression Machines
Tablet compression machines are essential in pharmaceutical manufacturing, but certain issues can arise during operation, potentially affecting product quality and production efficiency. Common problems include:
- Capping and Lamination – This occurs when tablets split or layer during compression. Causes may include improper moisture content in the granules, excessive compression force, or incorrect formulation.
- Weight Variation – Tablets may exhibit inconsistent weights, often due to irregular die fills, improper granule flow, or worn punches and dies.
- Sticking and Picking – Material adhesion to punches is typically caused by excessive binder, inadequate lubrication, or humidity affecting granule flow.
- Chipping and Cracking – Tablets may chip or crack due to insufficient hardness, improper machine adjustments, or inadequate granule size distribution.
- Machine Overheating – Overheating can result from prolonged operation at high speeds or inadequate lubrication of moving parts.
Troubleshooting Steps
- For Capping and Lamination:
-
- Adjust the moisture content of granules to the optimal level.
- Check and reduce compression and pre-compression force.
- Evaluate and modify the tablet formulation as needed.
- For Weight Variation:
-
- Inspect die fill mechanisms and enhance granule flow properties.
- Replace worn punches and dies.
- Calibrate the machine regularly to ensure consistent operation.
- For Sticking and Picking:
-
- Optimize the lubricant quantity and quality in the formulation.
- Maintain humidity levels in the manufacturing environment.
- Regularly clean punches and dies to avoid material build-up.
- For Chipping and Cracking:
-
- Increase tablet hardness by adjusting the compression force.
- Ensure even granule size distribution to promote uniform compression.
- For Machine Overheating:
-
- Schedule proper maintenance and lubrication of moving components.
- Operate the machine at recommended speeds and allow periodic cooling breaks.
By addressing these issues proactively and implementing adequate troubleshooting measures, manufacturers can enhance operational efficiency while ensuring the production of high-quality tablets that meet stringent regulatory standards.
Addressing Ejection Problems
When addressing ejection problems in tablet manufacturing, I would focus on several key factors. First, I would evaluate the ejection force applied during the process. Insufficient or excessive force can cause issues, so calibrating the equipment is critical. Next, I would examine the punch and die surfaces for any signs of wear, scratches, or residue buildup that could hinder smooth ejection. Ensuring proper maintenance and using a suitable coating on tools can mitigate these challenges. Additionally, I would analyze the tablet’s compressibility and hardness. Adjusting the formulation to optimize binding agents or lubrication can improve ejection reliability. By systematically addressing these variables, ejection issues can be effectively resolved, enhancing overall efficiency and product quality.
Troubleshooting Rotary Press Malfunctions
Key Considerations for Troubleshooting Rotary Press Malfunctions
1. Addressing Poor Tablet Weight Uniformity
To address issues related to inconsistent tablet weight, begin by verifying the granule flowability. The flow should be uniform to ensure consistency. Evaluate parameters such as the angle of repose (<40° is indicative of good flow) and bulk density range (0.3–0.8 g/cm³). Adjust feeder speed and paddle positioning as necessary to achieve uniform filling of dies. Regularly inspect feeder screws and reduce vibration in carrier mechanisms to maintain optimal flow.
2. Resolving Tablet Capping and Lamination
Capping and lamination often result from inadequate bonding during compression. Assess compression force and pre-compression settings, ensuring that pre-compression force ranges between 2–5 kN and main compression force does not exceed the material’s critical pressure. Additionally, consider modifying the formulation by increasing binder concentration or enhancing moisture content (target 2–4% residual moisture) to improve cohesiveness. Inspect punch condition and ensure die bore smoothness to prevent stress concentrators.
3. Minimizing Tool Sticking and Product Build-Up
Tool sticking issues are commonly due to improper lubrication or hygroscopic materials. To mitigate sticking, increase lubricant concentration within the recommended range (e.g., magnesium stearate at 0.2–1.0%) and ensure uniform distribution. Employ punch coating techniques, such as chromium or other low-friction materials, to create non-stick surfaces. Periodically check tooling cleanliness and ensure that relative humidity is controlled between 30–50% in the production environment.
4. Optimizing Tablet Ejection
Ejection difficulties stem primarily from excessive friction between the tablet edge and die wall. Key parameters to investigate include ejection stress, which should ideally be below 2 MPa. Regularly inspect ejection cams for wear and maintain a smoothly polished die surface to minimize resistance. If necessary, reformulate the tablet to optimize lubrication or reduce filler abrasiveness. Silicone-based or PTFE coatings on tooling may further enhance ejection smoothness.
5. Monitoring Critical Machine Parameters
Routine monitoring of critical machine settings is essential to avoid malfunctions. Key operational parameters include turret speed (range of 10–40 RPM depending on tablet size), punch penetration depth, and die table alignment. Use real-time monitoring systems to detect deviations and ensure that all settings remain within specified tolerances. Periodic recalibration of compression systems is also recommended to maintain consistency.
By addressing these technical parameters and systematically adjusting machine variables and formulation components, rotary press malfunctions can be effectively minimized.
Reference Sources
- Pharma Guideline: Working and Principle of Tablet Compression Machine
- Fluidpack: Working Principle of Tablet Compression Machine
- Chitra Mechtech: Tablet Press, Tablet Compression Machine – Working
- Wikipedia: Tablet Press
- Top Compression Testing Machine in China
Frequently Asked Questions (FAQs)
Q: What is the principle of tablet compression in a tablet compression machine?
A: The principle of tablet compression involves compressing powder into tablets using a compression machine, where the process involves applying pressure through compression rolls and punches to form hard tablets.
Q: How does a tablet compression machine work?
A: A tablet compression machine works by using punches and dies to compress powder into tablets. The powder is filled into a die cavity, then upper and lower punches compress the powder into a solid tablet.
Q: What are the main components of a tablet compression machine?
A: The main components of a tablet compression machine include the hopper, feeder, die cavity, punches (both upper and lower), and compression rolls or cylinders, which work together to shape and compress the powder.
Q: What is the role of compressive strength in tablet formation?
A: Compressive strength is critical in tablet formation as it determines the tablet’s ability to withstand mechanical stresses. The compression process involves assessing the material’s behavior under applied crushing loads to ensure optimal tablet integrity.
Q: How does an intermittent pneumatic compression machine differ from a tablet compression machine?
A: An intermittent pneumatic compression machine, such as a sequential compression device (SCD), is used primarily for medical purposes like preventing blood clots, whereas a tablet compression machine is designed to press powder into tablets.
Q: Can a universal testing machine be used in the tablet manufacturing process?
A: Yes, a universal testing machine can be used to test the compressive strength and material properties of powders and tablets, ensuring they meet required standards before mass production.
Q: What is the function of a leg compression machine in medical applications?
A: A leg compression machine, which uses pneumatic compression, helps prevent blood clots from forming by applying sequential pressure to the legs, thereby improving circulation.
Q: Why is understanding material properties important in the compression process?
A: Understanding material properties is essential in the compression process to predict how the material will behave under applied crushing loads, ensuring efficient tablet formation and quality.
Q: What factors affect the compression process in tablet manufacturing?
A: Factors affecting the compression process include the properties of the material, the compressive strength required, the design of the punches and dies, and the precise control of the compression machine work settings.
Q: How does the sequential compression device (SCD) aid in healthcare?
A: The sequential compression device (SCD) aids in healthcare by using pneumatic compression to apply sequential pressure to the limbs, which enhances blood flow and reduces the risk of blood clot formation.






